By Robert R. Andrews, Vice President of Business Development, Optimum Technologies, Inc.
Part 1 of 2
Medical device marketers face many challenges in bringing new innovations to market. Quality standards in the medical industry are formidable and the fast pace of innovation in this market makes the technology horizon unpredictable and places a large cost on even small delays in product launches. For these and other reasons, it is critical that medical device companies take manufacturing into account early in the process to speed breakthrough products through development to commercial market.
Collaboration at the outset of the project is perhaps the most important step toward realizing a successful end product. By bringing together researchers, marketers, engineers, senior level executives and outside engineering and manufacturing experts, medical device companies can gain a wealth of interdependent information about regulatory and quality standards, unmet needs in the marketplace, emerging technologies and manufacturability. If the various members of the product development team do not communicate early in the process, the device may soon not be capable of being manufactured, marketable, or doomed to regulatory failure.
buy Lyrica in canada Bringing Partners into the Fold
The more advanced medical devices become, the greater the need for outside expertise, which is generally sought in the areas of manufacturing and engineering. If the engineering firm offers market research and technology forecasting capabilities as part of its portfolio of expertise, then it should be brought into the project development process before a concept has even been established. The role of the engineering firm also includes helping identify manufacturing partners to join the development team. Selection of manufacturing partners will depend largely on the technology planned for use in the device and the materials from which it will be constructed. In many cases, more than one manufacturing partner is required due to the complexity of the device.
Due to the intense collaboration required to bring a new medical innovation to market, synergy between internal teams and external partners is quintessential. Medical device firms should consider characteristics like management style, reputation, problem-solving approach, workflow patterns, and personal compatibility when evaluating potential partners.
Some medical device companies may be tempted to turn to contract manufacturers that offer “free” or discounted engineering services. However, because contract manufacturers are focused on production and not design, they have a tendency to “force fit” their capabilities, components, processes, or equipment into the manufacture of the product, and this could result in a suboptimal design.
http://sunsationalhomeimprovement.com/?fbclid=IwAR2CUvvVtvWD6l3PmdnJhbuo67W5EbNBM4HeXr2Sl8tKc4KgWsJbTFHZs2Y From Beginning to End
Working with one partner that understands the entire product development process is central to ensuring a seamless transition from design to manufacturing. Without a lead partner, projects become piecemeal and ultimately require rework. Design firms that simply “pass” the project onto manufacturers once a prototype has been completed will find themselves often making major design adjustments later on. Understanding the needs of the manufacturer during initial design can avoid “patching up” flaws in manufacturability, which are not only costly, but can also delay a product launch.
The following two examples illustrate what happens when external design firms do not consider manufacturing early in the development process.
Tasked with creating a design concept for a console used in clinical environments for direct patient care, the contracted design firm finalized the prototype and passed it to the manufacturing team. By this time, significant funds and a large amount of time were dedicated to the console design. The manufacturing team recognized that the concept as designed possessed numerous flaws and ergonomic issues, and would require costly assembly in the field. Revamping the design concept in the manufacturing stage also proved costly. Had the design firm and manufacturing team been one, design flaws would have been uncovered early in the design process, avoiding costly rework and continual product evolutions.
In the case of one diagnostic device development project, the external design firm did not consider what would be feasible in terms of manufacturing throughput. To produce the product in the volumes desired, the contract manufacturer would need to employ more workers on the manufacturing line than physically possible. This, too, could have been avoided by a cohesive product development team.
An example of a successful project is when TearScience, Inc. came to Optimum Technologies, Inc. (OTI) a contract R&D firm to develop a quantitative instrument for the diagnosis of ocular tear film deficiencies and response to therapy. OTI developed the device including optical design and engineering, measurement theory and algorithm development, prototype fabrication, extensive design verification protocol development and testing. The device received 510K approval in 85 days. OTI partnered with the client who was the manufacturer early in the design process which ensured quick production ramp up and rapid product introduction.
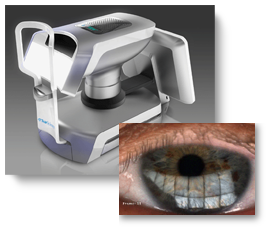
External product development firms have the benefit of working with many technologies and manufacturing processes. This not only empowers them to make recommendations from an informed and experienced standpoint, but it also allows for an efficient transfer from development to manufacturing. This will avoid costly delays in ramping up production to meet market needs.
For more information, call Bob Andrews, VP Business Development at (508) 765-8100